Editor's Note: This essay by Kimberly Chen and Matthew Herron was originally published in The Science Breaker on Sept. 10, 2019. It is reposted here with permission.
Discussions about the evolution of multicellularity tend to focus on animals and plants, but there have actually been at least 25 independent origins of multicellularity in the history of life on this planet, including fungi, slime molds, several groups of algae, cyanobacteria and myxobacteria. So how did early single cells evolve into organisms consisting of multiple cells, and why? What were the advantages of being a multicellular organism?
It would be helpful in answering these critical questions if we could study the fossil history of multicellular organisms. However, few fossils have been found that show the earliest stages of the transition to multicellular life. Most such transitions happened hundreds of millions or even billions of years ago, and fossils that old are very rare. So it is really hard to know just what happened that far back.
Since we couldn't learn much from fossils, we used experimental evolution to replay life's tape in the laboratory. One favored driver for the evolution of multicellularity is Predation. Because most predators can only consume prey up to a certain size, getting bigger can provide protection against being eaten, and one way for single-celled organisms to get bigger is to form multicellular structures.
We used single-celled, free-swimming green algae (Chlamydomonas reinhardtii) to explore the possible evolution of multicellularity. The predators we used in our experiment are filter-feeding ciliates (Paramecium tetraurelia). Despite being unicellular, these ciliates are larger and graze on small algae by sweeping them into their mouths with hairlike structures called cilia. We cultured some algae with predators and some without predators for a year to see if predators would increase the evolution of multicellularity.
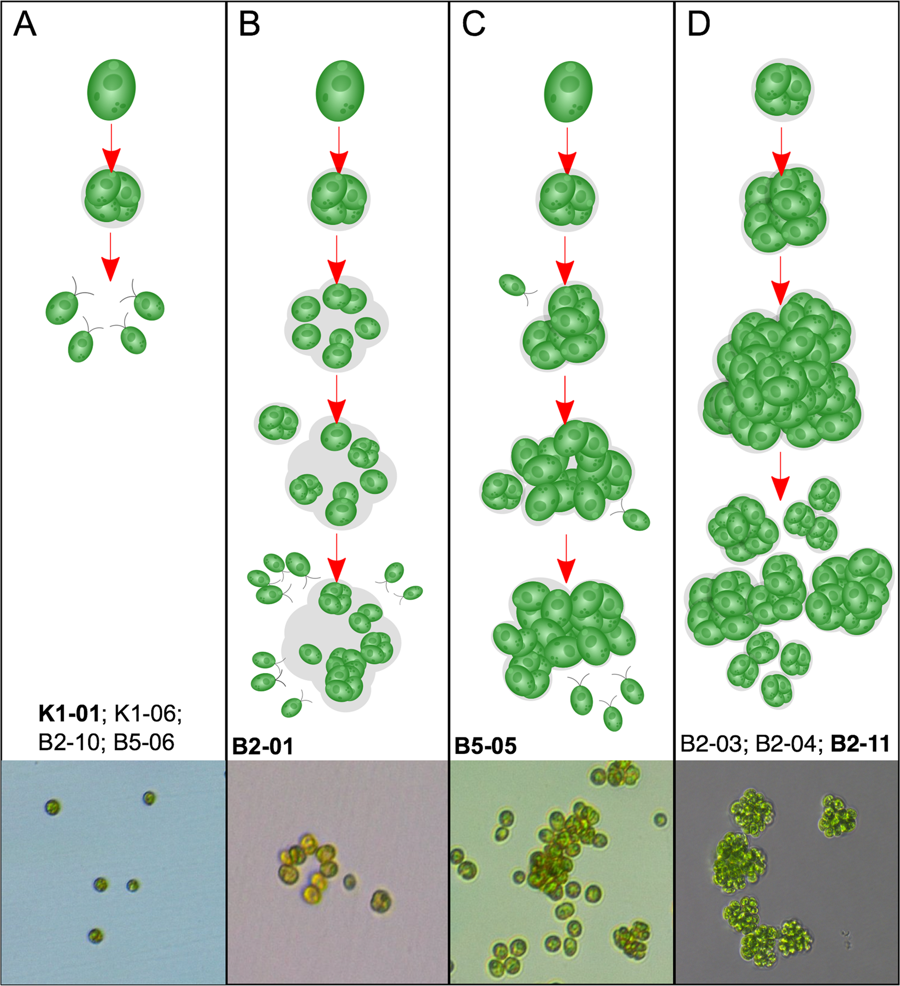
Single-celled algae normally multiply by a process called multiple fission, where a cell goes through one to three divisions to produce two, four or eight daughter cells. These daughters then hatch out of the mother cell wall to start the cycle again. By the end of our experiment, some of the cultures grown with predators had become multicellular by modifying their cell life cycle. In these evolved multicellular algae, we did not observe the last hatching step when the cell cycle is about to complete.
Instead, we found that each daughter cell continued its cell cycle within the mother cell wall, leading to multicellular clusters. Strictly speaking, cells in each cluster are descendants of a mother cell, and are genetic clones of each other. As clusters continue to grow bigger, they reach a limit and start to release single cells or small clusters of cells. In a separate experiment, we further showed that it is the cluster formation rather than other prey defenses that protects cells from predation. Selective pressure through predators, therefore, can favor the increase of clusters over single cells.
The multicellular life cycle is genetically fixed in the evolved multicellular algae, continuing even when they are grown in normal growth conditions without predators. But there is a price to pay. In nature, single-celled algae use slim threadlike structures called flagella to swim towards the light they need for photosynthesis. However, in the evolved multicellular algae each cell's flagella, even though they are present and active, are trapped within the mother cell wall. As a result, the multicellular clusters do not show noticeable movement. Such a drawback can be mitigated in the laboratory, since we culture these algae in an incubator with an ample supply of light. They might not be so lucky in nature.
From this experiment, we learned that multicellularity evolves readily in response to predation. This initial transition, although being a key step towards more complex life, does not seem to require organisms like green algae to evolve something new. Rather, this can be accomplished through a small modification to the existing cell cycle. The multicellular algae that evolved in our experiment also provide opportunities for further evolution experiments. For example, will they be able to regain the ability to swim? Can they evolve a division of labor, with cells becoming specialized to perform different tasks as we see in more complex multicellular organisms? These questions are under our current investigations.
Kimberly Chen is a postdoctoral researcher and Matthew Herron is a senior research scientist in the School of Biological Sciences.
For More Information Contact
A. Maureen Rouhi, Ph.D.
Director of Communications
College of Sciences